Neuromodulators Introduced in 2017
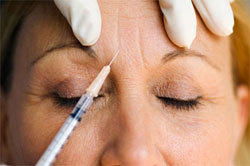
/Neuromodulators or botulinum toxins continue to gain popularity considering its effects on patients. Botox remains one of the most trusted brands in the market, and it does not seem to slow down along with the other two brands, Xeomin and Dysport. Of course, not everyone approves of having the botulinum toxins injected on them because of the effects that it has brought.
Revance Aesthetics is currently testing its RT002, and has had studies published regarding the efficacy of their neuromodulator, meanwhile EB-001 is fairly new but according to the company, their botulinum toxin seems to take effect much quicker than the current ones in the market. In that light, will the big neuromodulators brands be replaced by those still in testing phase?
In a previous article we published, we also wrote about Nabota, which has already been in use by some countries. Revance’s RT002 and Bonti’s EB-001 are currently undergoing trials, but both show potential.
According to Bonti, around 32% of patients show interest in having EB-001 injected on them. In a press release, the company mentioned they have conducted a clinical study, and it supports their claims about the product’s safety and efficacy. The product aims to be an introductory neuromodulator for those who have not tried and for time sensitive events.
As of December 2017, RT002 has reached Phase 3, which is showing better results than Botox. According to press releases, RT002 will have a six month duration period. If approved by the FDA, it’s the only neuromodulator that could ever receive that approval. Like the other neuromodulators, it has adverse effects like ptosis and headaches. Phase 3 will end its testing by February 2018.
These new brands will definitely shake the aesthetic market, since providers will have different options to choose. However, would these brands be able to replace the current ones?
These products will continue to compete regarding which one botulinum toxin would be enough to treat glabellar lines and crow’s feet, it is still unknown which among these treat these concern areas better. It is highly unlikely that the current three neuromodulators will be replaced, but possibly their market might change as there are other patients. Currently, patients can enjoy the present neuromodulators provided in your medical spa or practice.